mitä natriumbikarbonaatti tekee

Mitä on natriumbikarbonaatti?
Natriumbikarbonaatti tunnetaan myös nimellä ruokasooda. Se on valkoinen jauhe tai hieno kide, hajuton ja mauton, veteen liukeneva. Kemiallinen kaava on NaHCO3. Natriumbikarbonaatti on happosuola, joka muodostuu vahvan emäksen neutraloinnista heikon hapon kanssa, ja se on veteen liuotettuna heikosti emäksinen.
Kiinteä aine alkaa hajota yli 50 °C:n lämpötilassa natriumkarbonaatiksi, hiilidioksidiksi ja vedeksi ja hajoaa kokonaan 270 °C:ssa.
Natriumbikarbonaatin sovellukset ja käyttö:
Koska natriumbikarbonaatti on turvallista ja myrkytöntä, sitä käytetään usein laajalti tärkeänä elintarvikelisäaineena. Natriumbikarbonaatin käyttö on heikosti emäksistä, mikä voi neutraloida hapanta. Hajoamisen aikana syntyvällä hiilidioksidilla voi olla pörröinen rooli elintarvikkeissa. Natriumbikarbonaattia käytetään usein leivässä, kekseissä ja juomasoodassa.
Natriumbikarbonaatin tuotanto:
Natriumbikarbonaatin kemiallinen kaava NaHCO3 on tyypillinen epäorgaaninen yhdiste. Teollisessa tuotannossa on neljä päävalmistusmenetelmää:
- Ammoniakkipohjainen menetelmä:
Pääraaka-aineet: nestemäinen ammoniakki, vesi, hiilidioksidi.
Kemiallinen kaava CO2+NH3+H2O=NH4HCO3
NaCl+NH4HCO3=NaHCO3+NH4Cl
Tämä menetelmä vaatii huomiota: Koska NaHCO3 on liukoinen, NaHCO3 on saostettava, jotta NaCl:n ja NH4HCO3:n konsentraatio olisi suuri. Lisäksi, koska CO2:n liukoisuus veteen on pieni ja NH3:n liukoisuus veteen on suuri, NH4HCO3:n lisäämiseksi on ensin läpäistävä NH3 ja sitten syötettävä CO2.
- Yhdistetty alkalimenetelmä:
Kokonaisreaktioyhtälö:
NaCl + CO2+NH3+H2O=NaHCO3↓+NH4Cl (typpilannoite).
2NaHCO3=Na2CO3+H2O+CO2↑ (CO2:n kierrätys) (reaktio-olosuhteina lämmitys).
(NaHCO3 saostuu reaktiossa, joten tässä on saostumissymboli, mikä on tämän menetelmän kätevyys).
Ammoniakkipohjaisen menetelmän edut säilyvät ja sen haitat poistuvat, ja suolan käyttöaste nousee 96%:een. NH4Cl:ää voidaan käyttää typpilannoitteena (typpilannoitetta ei voi sekoittaa emäksisten aineiden kanssa, mutta sen puhtaus voidaan tarkistaa kasvien tuhkan avulla). Se voidaan yhdistää synteettisen ammoniakkilaitoksen kanssa raa'an ammoniakkikaasun CO:n muuttamiseksi CO2:ksi ja CaCO3:n poistamiseksi CO2:ksi mahdollisen ympäristön saastumisen vähentämiseksi.
- Synteesimenetelmä
Synteesimenetelmää kutsutaan myös soodamenetelmäksi. Tuotanto on usein yhdessä soodatehtaan tuotannon kanssa. Siksi synteettinen natriumbikarbonaatin tuotantoyksikkö on periaatteessa rakennettu soodatehtaan kanssa, jotta prosessin laskentajärjestelmästä saatavaa korkean lämpötilan soodaa voidaan hyödyntää suoraan äidin nesteytysprosessin nopeuttamiseksi. Tarjota pätevää lipeää myöhempiä toimintoja varten.
Sen varmistamiseksi, että emäliuoksessa oleva ylimääräinen NaHCO3 hajoaa kokonaan, NaHCO3:n kiteytymisen estyminen lipeän kuljetuksen aikana vähenee ja materiaalin korkea lämpötila säilyy koko toiminnan ajan. Siksi synteesimenetelmää kutsutaan usein korkean lämpötilan alkaliprosessiksi. Prosessin kulku on esitetty alla olevassa kuvassa.
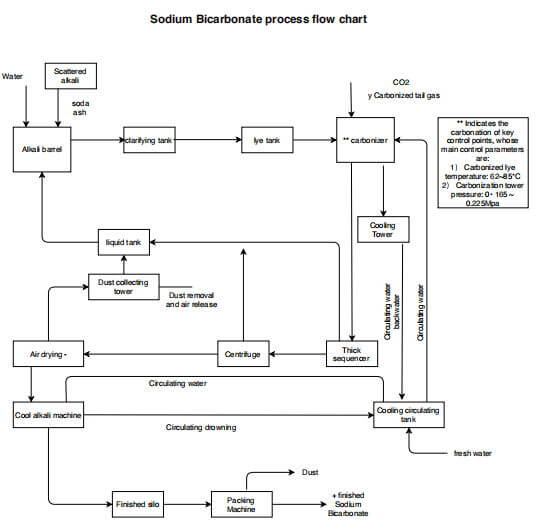
Natriumbikarbonaattiprosessin vuokaavio
- Trona-menetelmä
Tronan valmistusmenetelmää kutsutaan myös tronan hiiltymismenetelmäksi. Kiinalla on runsaat luonnonvarat tronaa. Karbonisointi (tarkalleen ottaen karbonisointi) on merkittävä tronan käsittelytekniikka Kiinassa. Tronan karbonisointimenetelmä tuottaa natriumbikarbonaattia. Sillä on keskeinen rooli maassa.
Tärkeimmät natriumbikarbonaatin tuottajat ja viejät:
Nykyään Kiinan ja Yhdysvaltojen osuus maailman natriumbikarbonaatin viennistä on suurin. Kiinaa pidetään viejänä, joka on kasvanut eniten.
Kiinan natriumbikarbonaatin tärkeimmät tuotantoalueet sijaitsevat Hunanissa, Sisä-Mongoliassa, Shandongissa ja Henanissa. Käytetyt tekniikat vaihtelevat hieman kunkin paikan luontaisten olosuhteiden mukaan. Esimerkiksi Sisä-Mongoliassa käytetään trona-menetelmää trona-soodan tuottamiseen. Shandong käyttää rannikkoetua yhdistetyn alkalimenetelmän ja synteettisen natriumbikarbonaattimenetelmän tuottamiseen.
Kiinan natriumbikarbonaatin vienti suuntautuu pääasiassa Japaniin, Etelä-Koreaan, Kaakkois-Aasiaan ja Lähi-itään. Yhdysvallat vie natriumbikarbonaattia Amerikkaan ja Eurooppaan.
Tuotantoon vaikuttavat tekijät:
Edellä esitettyjen tietojen perusteella on tärkeää mainita, että natriumbikarbonaatin hintaan vaikuttavat pääasiassa sellaiset tekijät kuin raaka-aineet (nestemäinen ammoniakki, soodasooda), kysynnän ja tarjonnan suhde sekä ympäristöystävällinen tuotanto.












